Carbon is one of the most common elements in nature, encompassing the properties of nearly all substances found on Earth. It exhibits a wide range of characteristics, such as varying hardness and softness, insulation-semiconductor-superconductor behavior, heat insulation-superconductivity, and light absorption-complete transparency. Among these, materials with sp2 hybridization are the main members of the carbon materials family, including graphite, carbon nanotubes, graphene, fullerenes, and amorphous glassy carbon.
Graphite and Glassy Carbon Samples
While the previous materials are well-known, let’s focus on glassy carbon today. Glassy carbon, also known as glassy carbon or vitreous carbon, combines the properties of glass and ceramics into a non-graphitic carbon material. Unlike crystalline graphite, it is an amorphous carbon material that is nearly 100% sp2-hybridized. Glassy carbon is synthesized by high-temperature sintering of precursor organic compounds, such as phenolic resins or furfuryl alcohol resins, under an inert gas atmosphere. Its black appearance and smooth glass-like surface earned it the name “glassy carbon.”
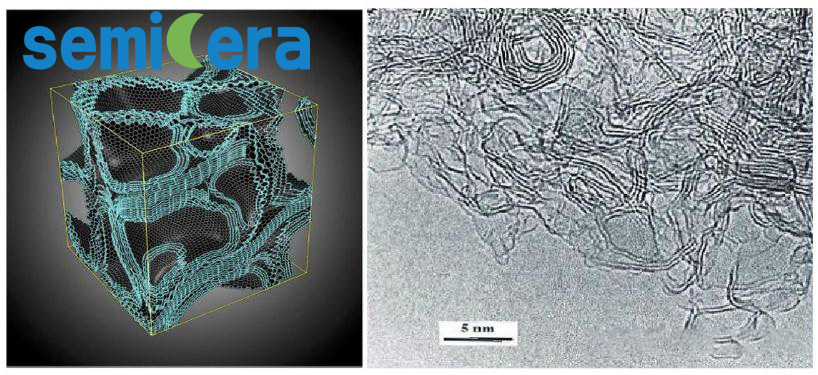
Since its first synthesis by scientists in 1962, the structure and properties of glassy carbon have been extensively studied and remain a hot topic in the field of carbon materials. Glassy carbon can be classified into two types: Type I and Type II glassy carbon. Type I glassy carbon is sintered from organic precursors at temperatures below 2000°C and consists mainly of randomly oriented curled graphene fragments. Type II glassy carbon, on the other hand, is sintered at higher temperatures (~2500°C) and forms an amorphous multilayered three-dimensional matrix of self-assembled fullerene-like spherical structures (as shown in the figure below).
Glassy Carbon Structure Representation (Left) and High-resolution Electron Microscopy Image (Right)
Recent research has found that Type II glassy carbon exhibits a higher compressibility than Type I, which is attributed to its self-assembled fullerene-like spherical structures. Despite slight geometric differences, both Type I and Type II glassy carbon matrices are essentially composed of disordered curled graphene.
Applications of Glassy Carbon
Glassy carbon possesses numerous outstanding properties, including low density, high hardness, high strength, high impermeability to gases and liquids, high thermal and chemical stability, which make it widely applicable in industries such as manufacturing, chemistry, and electronics.
01 High-Temperature Applications
Glassy carbon exhibits high temperature resistance in inert gas or vacuum environments, withstanding temperatures up to 3000°C. Unlike other ceramic and metal high-temperature materials, the strength of glassy carbon increases with temperature and can reach up to 2700K without becoming brittle. It also possesses low mass, low heat absorption, and low thermal expansion, making it suitable for various high-temperature applications, including thermocouple protection tubes, loading systems, and furnace components.
02 Chemical Applications
Due to its high corrosion resistance, glassy carbon finds extensive use in chemical analysis. Equipment made from glassy carbon offers advantages over conventional laboratory apparatus made from platinum, gold, other corrosion-resistant metals, special ceramics, or fluoroplastics. These advantages include resistance to all wet decomposing agents, no memory effect (uncontrolled adsorption and desorption of elements), no contamination of analyzed samples, resistance to acids and alkaline melts, and a non-porous glassy surface.
03 Dental Technology
Glassy carbon crucibles are commonly used in dental technology for melting precious metals and titanium alloys. They offer advantages such as high thermal conductivity, longer lifespan compared to graphite crucibles, no adhesion of molten precious metals, thermal shock resistance, applicability to all precious metals and titanium alloys, usage in induction casting centrifuges, creation of protective atmospheres over molten metals, and elimination of the need for flux.
The use of glassy carbon crucibles reduces heating and melting times and allows the heating coils of the melting unit to operate at lower temperatures than traditional ceramic containers, thereby decreasing the time required for each casting and extending the crucible’s lifespan. Moreover, its non-wettability eliminates material loss concerns.
04 Semiconductor Applications
Glassy carbon, with its high purity, exceptional corrosion resistance, absence of particle generation, conductivity, and good mechanical properties, is an ideal material for semiconductor production. Crucibles and boats made from glassy carbon can be used for zone melting of semiconductor components using the Bridgman or Czochralski methods, synthesis of gallium arsenide, and single crystal growth. Additionally, glassy carbon can serve as components in ion implantation systems and electrodes in plasma etching systems. Its high X-ray transparency also makes glassy carbon chips suitable for X-ray mask substrates.
In conclusion, glassy carbon offers exceptional properties that include high temperature resistance, chemical inertness, and excellent mechanical performance, making it suitable for a wide range of applications in various industries.
Contact Semicera for custom glass carbon products.
Email: sales05@semi-cera.com
Post time: Dec-18-2023